Well, welcome to the Huntington’s Disease Science Blog, a very personal web site dedicated to increasing awareness about this terrible disease, to attempt to dispel myths about research, scientific processes, scientists, and drug development, and to inform (you) of the latest attempts to understand and treat HD. But before we commence with my regular (probably biweekly) reviews of recent findings, I wanted to make a few comments about our work (that of the scientists and physicians trying to solve this very difficult problem). Our goal is to improve the lives of people and families affected by HD, and we hope that out small contributions will bring forth a treatment (or better yet, a cure) for HD, so that future generations will be spared of a life struggling with the decline brought forth by this (thus far) incurable, inherited, disease.
Science’s goal is to “disprove hypothesis” – not to prove them. The ‘theories’ that are prevalent are the ones that have withstood the test of time (so far) – people viciously try to bring them down, but they can’t: the evidence argues that these theories best fit available evidence. This is an important concept: it explains why scientists are always argumentative, and are apparently hard at work to bring someone else’s work to the ground. This is how our knowledge builds. We (scientists) acknowledge that we should only make claims based on available evidence. What seems correct today might not be applicable tomorrow, as new facts are described which change our understanding of a process or an observation. So we can never claim ‘we are right’, or ‘this is the way it is’… however, just because we accept our limitations, this does not mean that anything else is possible! Note, for example, the debate between evolution and ‘intelligent creation’…. Accepting that we might be wrong (unlikely in this case) does not immediately imply that any other (unsubstantiated theory) must be right or holds equal ground… we still base our statements on the best available evidence. Show scientists evidence of an alternative explanation, and undoubtedly and very quickly, most scientists will jump ship and trash the work of those (always the others) who got it wrong!
Let me again reiterate an important fact: scientists are human beings - we make mistakes, we are often wrong. Society sometimes forgets this fact and assumes that scientists should have answers for everything… Even though I am a scientist, I acknowledge there are areas where scientists should not mess with…. Stick to your area of expertise!

Let me tell you a bit about publishing… when we publish our work, typically we have to write a story that is cohesive and complete (especially for ‘high impact’ journals such as Science, Nature, Cell, Neuron, etc). Often times this leads to the (wrong) perception that we really understand what is happening. This is seldom the case, even though eventually the cumulative efforts of many people will yield some answers – of that you can be certain: we have learned a lot about this disease, but not enough yet. So hang in there with us, we are all walking together in the same direction. It is simply a long, arduous road.
The nature of scientific peer-review publishing is such that unless one is able to offer a report which leaves out ambiguities, it will have a tough time getting published. This is very unfortunate because some of the ‘loose ends’ are the interesting ones, and it is through the ambiguities in the work that arise important questions about the main hypothesis driving the work. I have often times found myself torn between including data I could not fully explain, or excluding it for the sake of publication. As the livelihood of most scientists working in academia relies on publishing, the decision invariably leads to not including those results.

Drug discovery is a tricky business. It takes on average almost a billion US dollars and over ten years of work to market a drug…. These are average figures: it is thought to be even more costly and lengthy for mental indications. So when you read a paper suggesting that a ‘treatment’ has been identified that works well in mice or rats, adjust your expectations!
Most of the time many of these positive results cannot be replicated, and even if they are, and assuming the biology is conserved between the disease states in the rodent and in people, it would still take many years to make as drug to treat people. I want to illustrate some of the steps that are required in drug discovery before a drug can be approved by the FDA (USA) or EMEA (European Union)… fasten your belts, as it is a bumpy and lengthy ride!!
The initial period (usually 1-3 years) involves identifying a mechanism or a single molecular target (an enzyme, or a receptor, for instance) which might positively affect some aspect of the disease process. For instance, in the case of HD, the identification of the gene causative for the disease (termed huntingtin, or IT-15), can be the focus of developing therapies aimed at modulating its function (or in this case, eliminating the mutated protein). In the case of huntingtin, developing a small molecule chemical to restore its function to a ‘normal state’ is not possible, as huntingtin is not a protein which can be modulated via small molecules. In other instances, say in the case of finding an enzyme which can be inhibited or activated, a chemical campaign is initiated to find molecules with the right activity. For example, if we take the example of the serotonin reuptake inhibitors (SSRIs) used to treat depression, small molecule chemicals were developed which inhibit its function. In this case, the serotonin reuptake transporter is a protein whose role is to take serotonin, a neurotransmitter, from the synaptic medium (outside the cells), and bring it inside of cells. This was based on the observation that there was a decrease of serotonin (or 5-HT, 5-hydroxytryptamine, a chemical neuro-transmitter) in people suffering from unipolar depression.
The development of such chemicals is slow: it involves finding a molecule, which can act at the protein target in a potent and selective way (that is, it does not interact with other related proteins). This typically takes several years, as the molecules have to be optimized to be selective and safe.
The field of drug metabolism and pharmacokinetics (DMPK) is needed to assess that the chemical can effectively reach the protein target (in this case, brain neurons) at a concentration effective to inhibit this process. If you think about it, this is not simple: an animal must take a chemical (preferably through oral administration), the chemical must make it outside of the gastrointestinal tract, get into the blood stream, travel to the brain (most chemicals cannot get into the brain as the brain/blood barrier (BBB) prevents most chemicals from reaching the neurons in the brain), and interact with the target in question. Most importantly, the chemical must do this without negatively affecting other mechanisms (hence not causing toxicity). The development of a safe and effective chemical is a very difficult task and it involves the work of many chemists and biologists working together very closely. The teams involved in drug discovery involve chemists (medicinal and synthetic chemists) and biologists (pharmacologists, molecular biologists, neuroscientists, experts in animal behavior and other disciplines).
After animals are given the chemical, we must assess not only that the drug is doing what is supposed to (in the case of SSRIs, increase serotonin levels), but we must also assess that increasing serotonin levels leads to a biological effect which we think will be of benefit to the disease in question. This sounds easy but it is hard to do: often times we do not know whether the brains of animals used in research (mostly mice and rats) respond in the same way that human brains do. Therefore, a large area of research is to develop animal models of disease. In the case of HD, we have developed mice and rats genetically engineered to carry the human mutations. These animals develop a progressive degenerative process which we feel represents a state similar to some aspects of the human condition. Although imperfect, these models help us in deciphering the mechanisms which might be affected in the brains of people suffering with HD, and act as ‘models’ to assess whether drugs are effective.
After we have shown that the chemical does what is supposed to, we invest a lot of time in improving its characteristics in terms of potency and safety. We must ascertain that a chronic administration of the molecule (over months) does not pose any safety issues (such as liver damage, the most common source of safety issues since the liver is the organ which breaks down most chemicals in the body). The safety studies required to get approval to initiate a clinical study (in people) take at least 12 months (these are called IND enabling studies; IND= investigational new drug).
The process of the clinical trials takes on average more than 5 years (with few exceptions), since drugs must be tested in unaffected individuals (to assess safety, as in Phase I trials), or in affected people (Phase II) and to determine a dose which is predicted to be effective, but without reaching doses which might pose a risk in terms of toxicity. The Phase III trials are the most costly as they involve a large number of patients and they take a long time to complete (typically 1-2 years from initiation of the trial; this does not account the time it takes to get all the patients enrolled and all the paperwork required for the hospitals and clinical centers to be ready to start a study of this magnitude!)
Typically, most drugs these days (especially for mental indications) fail in the clinic because we got the biology wrong. The drug is safe (somewhat) but it does not yield the expected improvement in clinical symptoms. This is probably because we did not understand the disease properly, the animal models did not accurate mimic the human disease, or we had the wrong hypothesis.
One of the areas that requires more work (and hence an area where all of you can help) is gathering more information from the patients suffering from the disease.
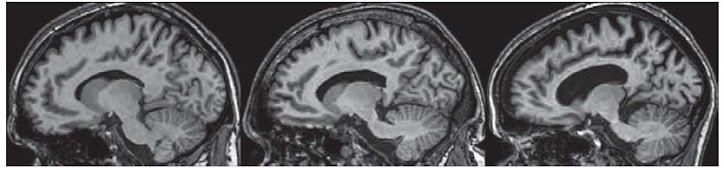
After all, we are trying to cure the human disease. Yet, due to ethical and societal issues, we know least about the biology of the human species. We tend to know a lot more about how the brains of mice and rats work, that those of people. This is a significant reason why we got things wrong often. If there were more people participating in clinical research (through imaging their brains, for instance), we might be able to develop better theories. At my work, we try to gather as many observations as possible from people suffering from HD, so that we can assess if the models we have developed mimic the disease. Your help in participating in observational trials (no drugs being taken) helps tremendously in gathering such data, and refining our understanding of the disease.

I hope these words are helpful in understanding some basic concepts about the work we do.

.jpg)
typo: "work, that those of people" => "work than those of people"?
ReplyDelete